From Snapshots to Landscapes: Mapping Molecular Dynamics Across Conditions
Molecular systems are in constant motion, yet our experimental tools are often limited to capturing single, static moments. This leaves critical parts of the story in the dark. In this post, we explore an approach that turns these scattered 'snapshots' into a connected, interpretable landscape.

Stills Versus Movie
High-resolution techniques like cryo-EM, NMR, and AFM give us exquisitely detailed stills—atomic-level portraits of a sample at a single instant in time. But trying to understand a dynamic system from a handful of frames is like piecing together a movie from a few high-definition screenshots.
Without the sequence between them, we lose the sense of motion: how one conformation morphs into another, where the turning points lie, and which fleeting intermediates might pass by, completely unseen.
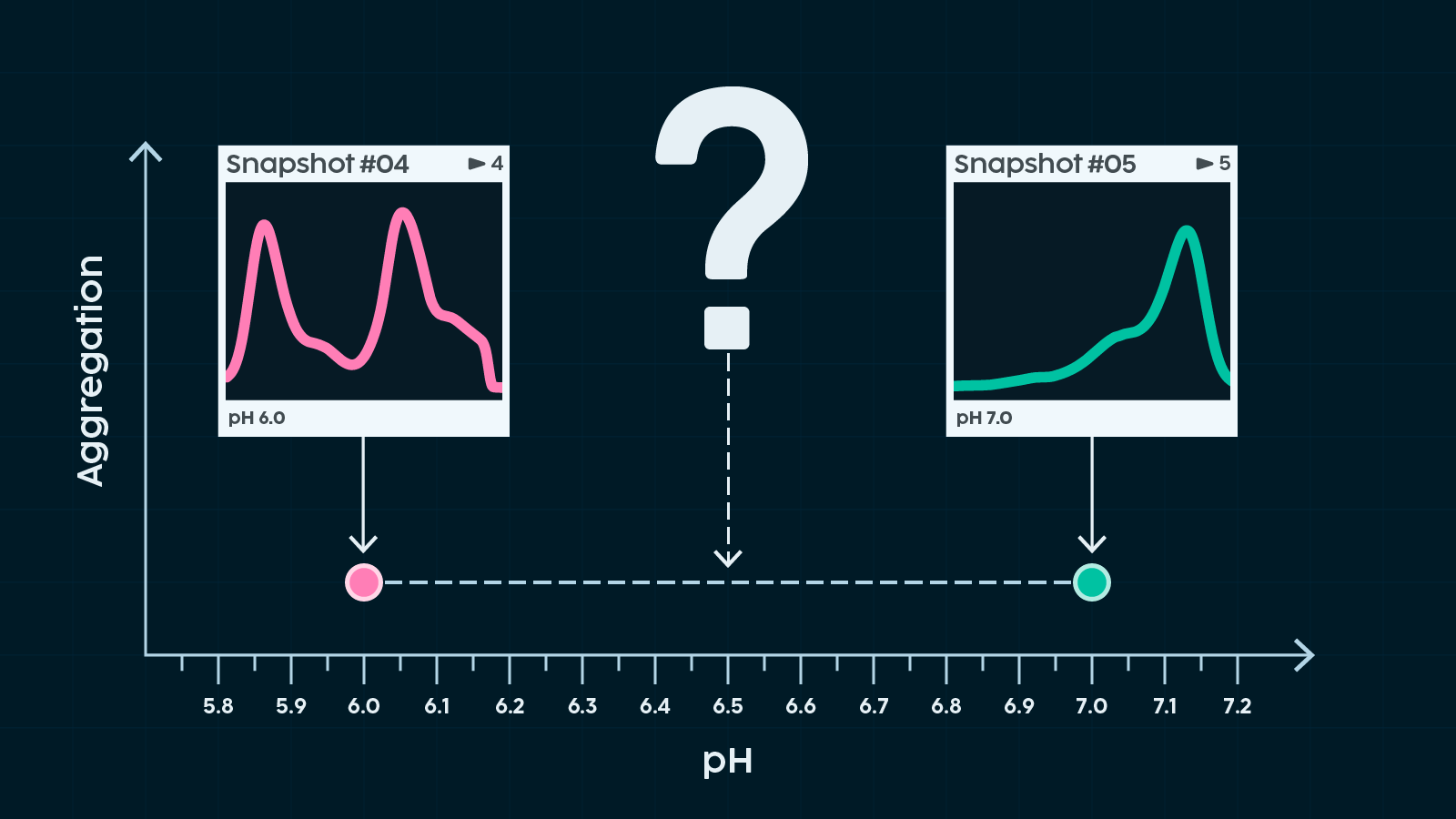
It's a familiar story. We might observe that a sample exists in two very different states under separate conditions—say, depending on pH or temperature. By digging deep, we can learn a great deal about each state, yet the journey between them remains a mystery. How do we get from A to B? Where does the transition actually occur? Is it reversible? Are there crucial intermediates we’re missing?
Ideally, we would generate a large number of samples and analyse all of them across the condition landscape. In practice, that quickly becomes untenable. High-resolution methods are costly in both resources and time, and beam-time is notoriously scarce.
Challenges like this are everywhere—in the toxic aggregation cascades of neurodegenerative disease, the shifting microenvironments that drive cancer metastasis, and the complex formulation of biologic drugs.
Building the Narrative Between the Stills
To give our high-resolution data points real context, we need a way to link them together—ideally with a more accessible and scalable method. This requires a readout that changes in a meaningful way as the sample transitions between states. For example, the twists and turns of aggregation can be tracked using light scattering or turbidity.
We also need a way to traverse that condition space in a controlled manner. You can do this by preparing a large number of samples with slightly different conditions, or by making well-defined, incremental changes to a single sample. The latter approach has a major advantage: as the sample evolves, any change in the readout can be directly linked to the changing condition. It's a simple way to eliminate nagging concerns about sample-to-sample variability.
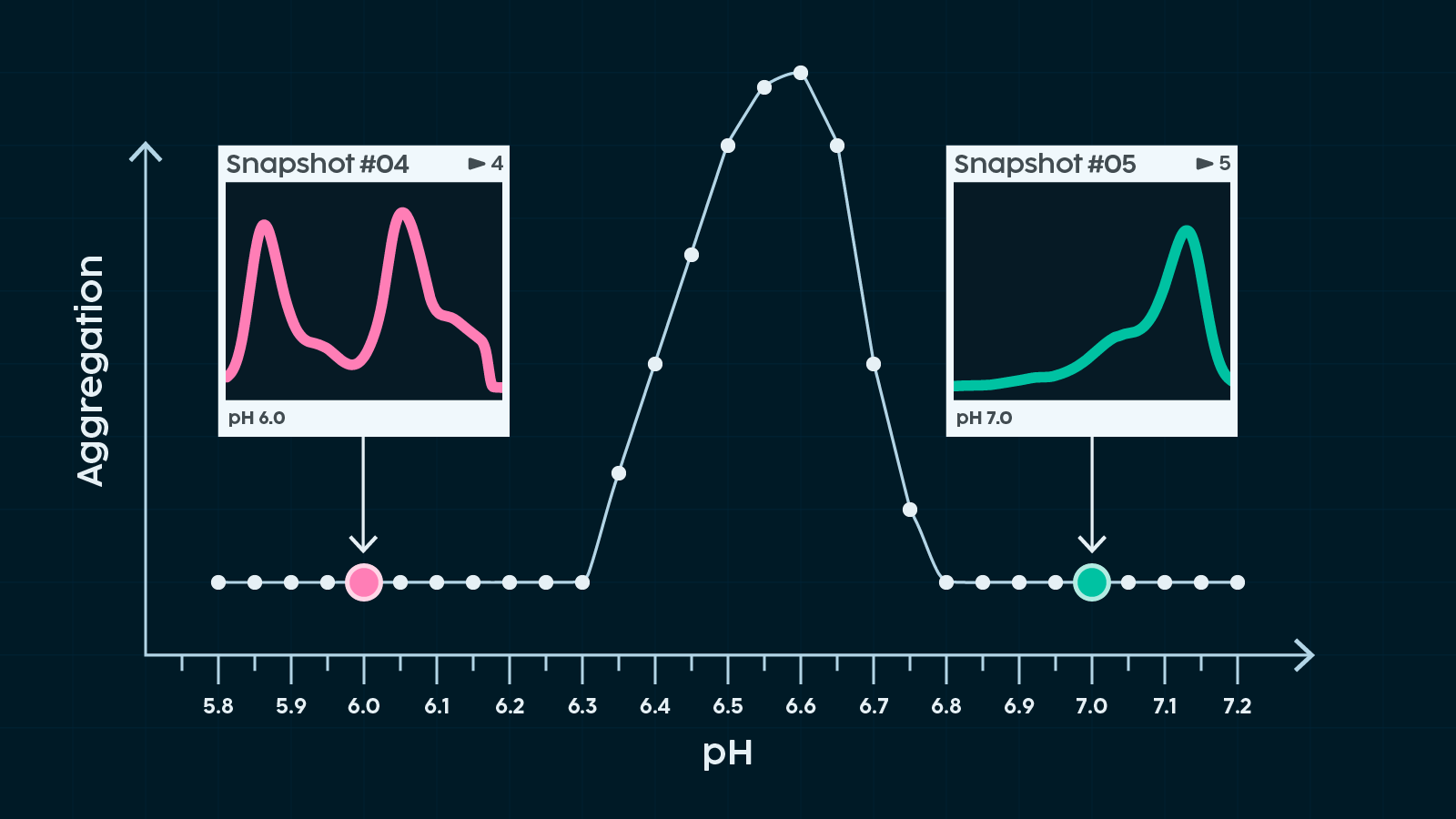
With a continuous readout, we can map out the space between our data points in detail and finally start to answer some of the questions we posed earlier. This is especially powerful for pinpointing exactly where along a condition gradient a transition takes place.
Filling Out the Landscape
But we can go further than just connecting scattered points. By working systematically, we can use these first explorations as a starting point to map out a much broader swath of the condition landscape.
This wider overview helps us link insights from different experiments with far greater confidence. Instead of treating each high-resolution dataset in isolation, we can place them within a shared framework—a coordinate system for sample behaviour.
Let's be honest: combining data from different experiments, often performed in different labs, on different instruments, at different times, can be a nightmare if the broader context is missing.
Since the landscape is often vast and dynamic, even small changes in conditions (buffer strength, pH, temperature, sample age, etc.) can shift the state we're analysing. Without continuous monitoring, these changes can be surprisingly easy to miss.
The Power of Zooming Out
When we're deep in research, it's tempting to zoom in on the most interesting details. Controlling variables and extracting high-resolution data are essential tools for discovery. But when it comes to complex, dynamic systems, clarity doesn't always come from getting closer. Sometimes, it comes from stepping back.
If we want to truly understand how our samples behave, we need to make space for methods that let us see the whole story: how conditions change, how transitions unfold, and how all the pieces fit together over time.
Adopting this perspective is more than a methodological tweak. It's a commitment to a different experimental philosophy, one that prioritises the dynamic journey of a molecule over its static endpoints.
It's about making the choice to see the entire movie, not just a collection of beautiful, but disconnected, stills. For me, this shift in perspective became an obsession. It’s what drove me from the lab bench to the workshop, combining my background in science and engineering to build a tool that could answer the questions I couldn’t tackle otherwise. It’s the principle that our team is built on today.
So, the next time you're preparing to dive deep into structure, assembly, or interactions, it's worth asking yourself:
Do I really know what’s happening between the data points?
Could a simple overview save me a costly misstep, or better yet, reveal something entirely new?
More Reading

Exploring the Role of Liquid-Liquid Phase Separation in Cellular Functions



